Background
In unfit patients (pts) with newly diagnosed (ND) AML, long-term survival remains short (mOS 14.7 months) despite improved responses (CR 37% and CR/CRi 66%) with azacitidine (AZA) and venetoclax (VEN) (VIALE-A, DiNardo NEJM 2020). In a pooled analysis of AZA-VEN in ND AML pts with poor risk cytogenetics the response rates were higher in TP53wt pts (CR/CRi 70%) compared with TP53mut pts (CR/CRi 41%) (Pollyea Clin Cancer Res 2022). The measurable residual disease (MRD)-negative rate was 41% in AZA-VEN treated pts in VIALE-A which was associated with improved survival (Pratz JCO 2022). Pivekimab sunirine (PVEK, IMGN632) is a first-in-class antibody-drug conjugate (ADC) comprising a high-affinity CD123 antibody, cleavable linker, and an indolinobenzodiazepine pseudodimer (IGN) payload. The novel IGN payload alkylates DNA and causes single strand breaks without crosslinking (Kovtun Blood Adv 2018).
Methods
This is an ongoing, open-label, multicenter, Phase 1b/2 study of PVEK in combination with AZA +VEN in adults with ND CD123-positive AML (any CD123+ expression by local flow cytometry or IHC). Pts received the recommended phase 2 dose of PVEK 0.045 mg/kg IV on D7 + AZA 75 mg/m 2 SC or IV daily on D1-7 + VEN up to 400 mg PO daily for 14 to 28 days (based on cohort assignment) in a 28-day cycle. Bone marrow assessment was performed at D14 (cohort 1) or D21 (cohort 2) of cycle 1 to determine VEN duration. The primary endpoints are composite CR rate (CCR [CR+CRh+CRp+CRi]), MRD rate (assessed centrally [Hematologics, Inc.] by flow cytometry; <0.1% defined as negative) and duration of remission. Key secondary endpoints are safety, pharmacokinetics and immunogenicity. Responses were determined using ELN 2017 criteria (with the addition of CRh) and a 14-day post-marrow count recovery window.
Results
As of July 17, 2023, data is available for 50 ND pts (n=25 per cohort) treated with PVEK+AZA+VEN and are aggregately reported here. The median age was 74 years (range, 46-83), 42% were ≥ 75 years old, 26% had secondary AML, 64% were ELN 2017 adverse risk, and 38% had a TP53mut.
The most common non-hematologic treatment-emergent adverse events (TEAE) (all grades [grade 3+]) seen in > 20% of all pts were febrile neutropenia (44% [38%]), constipation (42% [2%]), peripheral edema (36% [4%]), diarrhea (36% [2%]), hypophosphatemia (32% [0%], nausea (28% [4%]), and hypokalemia (24% [2%]). No veno-occlusive disease (VOD) events were reported. In responders, the median time to achieve absolute neutrophil count ≥500/µL and platelet count ≥50,000/µL was 35.5 days (range, 20-55) and 22 days (range, 20-55), respectively. The median post-remission cycle delay (beyond cycle day 28) was 14 days. Two pts discontinued PVEK due to a TEAE (edema; marrow aplasia). The 60-day mortality was 4%.
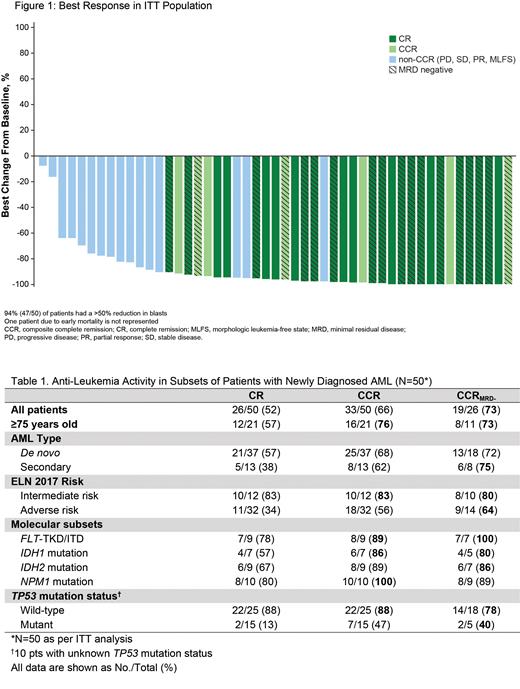
In the ITT population (N=50) the CR rate was 52% (26/50), and the CCR rate was 66% (33/50). In pts ≥ 75 years old, the CCR rate was 76% (16/21). In pts known to be TP53wt, both the CR and CCR rate was 88% (22/25). Additional response subsets in Table 1. Twenty-one pts received 9-14 days of VEN, 9 pts received 15-21 days of VEN and 20 pts received ≥ 22 days of VEN in cycle 1; of those who received ≤ 14 days of VEN the CR rate was 71% (15/21) and the CCR rate was 76% (16/21).
Of the 26 pts who achieved CCR and had an MRD-evaluable sample, 73% (19/26) achieved MRD negativity (CCR MRD-, <0.1% residual blasts); notably all had undetectable MRD levels (lower level of detection is 0.02%). MRD-negativity was achieved rapidly with a median of 1.7 months (range, 0.8-4.5). CCR MRD- rates were high across major molecular subsets, including FLT3 (ITD/TKD) at 100% (7/7), IDH1/2 at 89% (8/9), and NPM1 at 89% (8/9), as well as ELN risk groups (intermediate 80% [8/10]; adverse 64% [9/14]).
Conclusion
With a manageable safety profile in pts with ND AML, the PVEK+AZA+VEN triplet demonstrated anti-leukemia activity with robust CR rates and early MRD negative responses in a cohort enriched for adverse disease characteristics (64% ELN adverse risk; 38% TP53mut). Encouraging CCR MRD- rates were observed across cytogenetic/molecular subsets, and the majority of responding pts achieved early and deep remissions, which may translate to improved clinical outcomes. The regimen was well tolerated with no new safety signals, and the addition of PVEK to the AZA-VEN backbone did not appear to meaningfully prolong count recovery.
Disclosures
Daver:ImmunoGen: Consultancy, Research Funding; Syndax: Consultancy; Jazz: Consultancy; Novimmune: Research Funding; FATE: Research Funding; Agios: Consultancy; Shattuck Labs: Consultancy; Novartis: Consultancy; AbbVie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Trovagene: Research Funding; Astellas: Consultancy, Research Funding; Kite, a Gilead company: Consultancy, Research Funding; Kronos Bio: Research Funding; Servier: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Glycomimetics: Research Funding; AROG: Consultancy; Hanmi: Research Funding; Celgene: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding. Montesinos:Jazz pharma: Consultancy, Research Funding, Speakers Bureau; Kura oncology: Consultancy; Astellas: Consultancy, Speakers Bureau; Takeda: Consultancy, Research Funding; GILEAD: Consultancy; Abbvie: Consultancy, Research Funding, Speakers Bureau; Menarini-Stemline: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; INCYTE: Consultancy; NERVIANO: Consultancy; Pfizer: Consultancy, Research Funding, Speakers Bureau; OTSUKA: Consultancy; Janssen: Speakers Bureau; BMS: Consultancy, Other, Research Funding; BEIGENE: Consultancy; Ryvu: Consultancy; Celgene: Consultancy; Daiichi Sankyo: Consultancy, Research Funding. Altman:Stemline Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kymera: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BioSight: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Curio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kura Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Consultancy, Research Funding; ALX Oncology: Consultancy, Research Funding; Bluebird Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Fujifilm: Consultancy, Research Funding; Kartos Therapeutics: Consultancy, Research Funding; Loxo: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Telios: Consultancy, Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Syros: Consultancy, Membership on an entity's Board of Directors or advisory committees; MD Education: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amphivena: Consultancy, Research Funding; Aprea AB: Consultancy, Research Funding; Aptose Biosciences: Consultancy, Research Funding; Boehringer Ingelheim: Consultancy, Research Funding; GlycoMimetics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cyclacel: Consultancy, Research Funding; Immunogen: Consultancy, Research Funding. Wang:Gilead: Consultancy; GlaxoSmithKline: Consultancy; Jazz: Consultancy; Kite: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; PharmaEssentia: Consultancy; Takeda: Consultancy; Dava oncology: Speakers Bureau; Kura Oncology: Speakers Bureau; BMS: Consultancy; Astellas: Consultancy, Speakers Bureau; Abbvie: Consultancy. Roboz:Blueprint: Consultancy; Bluebird bio: Consultancy; Actinium: Consultancy; AZ: Consultancy; Astellas: Consultancy; Amgen: Consultancy; BMS: Consultancy; GSK: Consultancy; Janssen: Consultancy, Research Funding; Jasper: Consultancy; Jazz: Consultancy; MEI: Consultancy; AbbVie: Consultancy; Agios: Consultancy; Mesoblast: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Syndax: Consultancy; Takeda: Consultancy. Begna:Immunogen: Research Funding; MEI Pharma: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees. Vyas:Pfizer: Honoraria; Gilead: Honoraria; BMS: Research Funding; Auron Therapeutics: Current holder of stock options in a privately-held company; Astellas: Honoraria; Jazz: Honoraria; Abbvie: Consultancy, Honoraria. Platzbecker:Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; medical writing support, Research Funding; Merck: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Celgene: Honoraria; Amgen: Consultancy, Research Funding; Syros: Consultancy, Honoraria, Research Funding; Janssen Biotech: Consultancy, Research Funding; Jazz: Consultancy, Honoraria, Research Funding; Servier: Consultancy, Honoraria, Research Funding; Silence Therapeutics: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Geron: Consultancy, Research Funding; AbbVie: Consultancy; Curis: Consultancy, Research Funding; MDS Foundation: Membership on an entity's Board of Directors or advisory committees; Fibrogen: Research Funding; Roche: Research Funding; BeiGene: Research Funding; BMS: Research Funding. Burke:DAVA Pharmaceuticals: Other: Travel, Accommodations, Expenses ; Takeda: Research Funding; Novartis: Research Funding; Seagen: Consultancy; Lilly: Consultancy; Eisai: Consultancy. Walter:Amgen, Aptevo, Celgene, Janssen, Jazz, MacroGenics, Pfizer: Research Funding; Abbvie, Adicet, Amphivena, BerGenBio, Bristol Myers Squibb, GlaxoSmithKline, Orum: Consultancy; ImmunoGen, Jura: Consultancy, Research Funding. Advani:Seattle Genetics: Research Funding; Glycomimetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Immunogen: Research Funding; Macrogenics: Research Funding; Kura: Honoraria; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Beam: Honoraria; Servier: Research Funding; Nkarta: Honoraria; Taiho: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite: Honoraria, Other: consulting, Research Funding; Incyte: Research Funding; OBI: Research Funding; Pfizer: Honoraria, Research Funding; Amgen: Honoraria, Other: advisory board, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Sallman:AbbVie, Affimed Gmbh, Gilead, Incyte, Intellisphere, LLC, Molecular Partners AG, PGEN Therapeutics, Inc., Takeda, Zentalis; Advisory board for AvenCell, BlueBird Bio, BMS, Intellia, Jasper Therapeutics, Kite, Magenta Therapeutics, NKARTA, Novartis, Orbita: Consultancy; Aprea, Jazz: Research Funding. Pemmaraju:Curio Science: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSA Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Neopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Aplastic Anemia & MDS International Foundation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CancerNet: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CareDx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dava Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Harborside Press: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Imedex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Intellisphere: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Magdalen Medical Publishing: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pacylex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Medscape: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Menarini Group: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; OncLive: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Patient Power: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PeerView Institute for Medical Education: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Physician Education Resource (PER): Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASH Committee on Communications: Other: Leadership; ASCO Cancer.Net Editorial Board: Other: Leadership; Karger Publishers: Other: Licenses; United States Department of Defense (DOD): Research Funding; National Institute of Health/National Cancer Institute (NIH/NCI): Research Funding; Stemline: Consultancy, Membership on an entity's Board of Directors or advisory committees; Protagonist Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; ImmunoGen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ClearView Healthcare Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Cimeio Therapeutics AG: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aptitude Health: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; HemOnc Times/Oncology Times: Other: Uncompensated; Bristol Myers Squibb Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Abaza:Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Rigel: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; Biomea: Research Funding; Curis: Research Funding; Biosight: Research Funding; ALX Oncology: Research Funding. Kantarjian:Abbvie: Consultancy, Honoraria; Amgen: Honoraria; AstraZeneca/MedImmune: Honoraria; Daiichih-Sankyo (Inst): Honoraria, Research Funding; Immunogen (Inst): Honoraria, Research Funding; Ipsen: Honoraria; Jazz Pharmaceuticals (Inst): Honoraria, Research Funding; KAHR Medical: Honoraria; Novartis: Honoraria; Pfizer: Honoraria; Precision Biosciences: Honoraria; Shenzhen Target Rx: Honoraria; Taiho Pharmaceutical: Honoraria; Abbvie (Inst): Research Funding; Amgen (Inst): Research Funding; Astellas Pharma: Honoraria; Bristol-Myers Squibb (Inst): Research Funding; Ascentage Pharma (Inst): Research Funding; Ascentage Pharma Group: Honoraria; Novartis (Inst): Research Funding. Oshrine:ImmunoGen, Inc.: Current Employment, Current equity holder in publicly-traded company. Du:ImmunoGen, Inc.: Current Employment, Current equity holder in publicly-traded company; Incyte: Current equity holder in publicly-traded company; Nektar Corporation: Current equity holder in publicly-traded company; Daiichi Sankyo: Ended employment in the past 24 months. Malcolm:ImmunoGen, Inc.: Current Employment. Sweet:Incyte: Research Funding; Nkarta: Consultancy; Jazz Pharmaceuticals: Consultancy, Research Funding; Daiichi Sankyo: Consultancy; Mablytics: Consultancy; Pfizer: Consultancy; Curis: Consultancy; Novartis: Consultancy; Arog: Consultancy; BerGenBio: Consultancy; Astellas: Consultancy; Bristol Myers Squibb: Consultancy; Gilead: Consultancy; BeiGene: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal